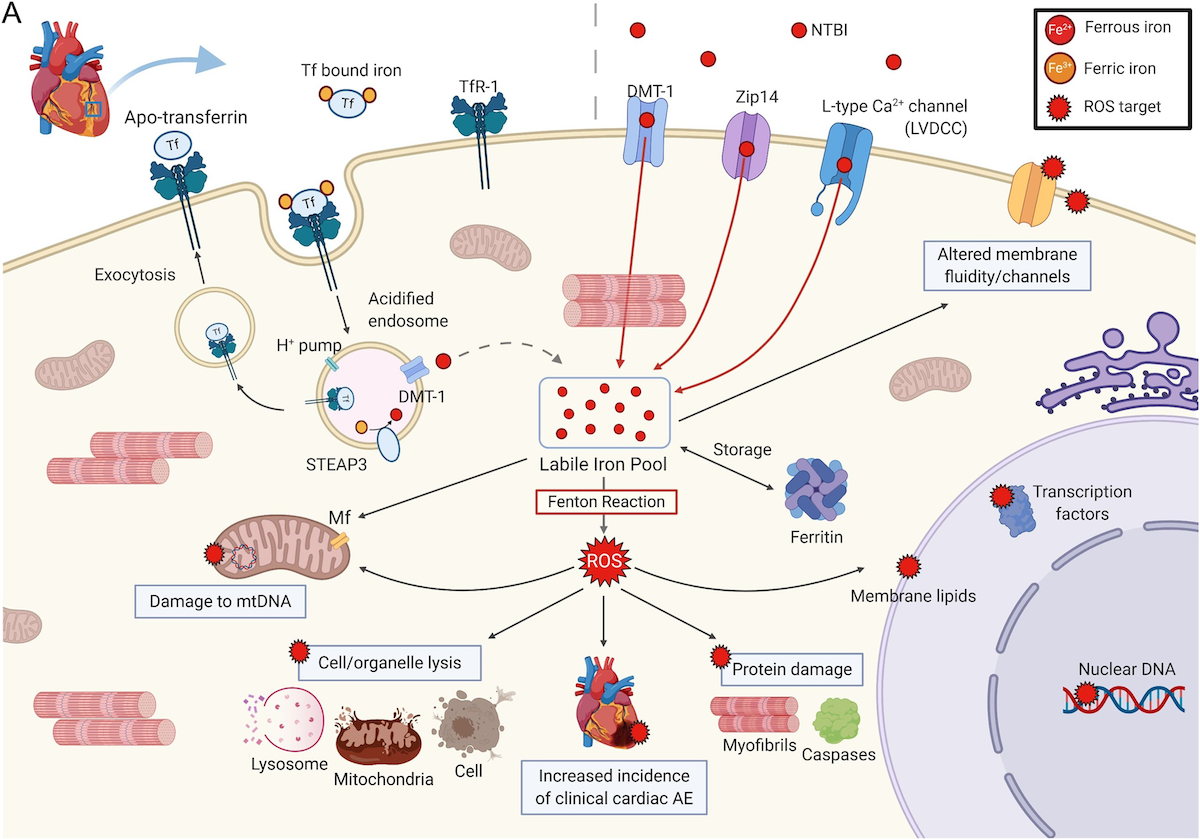
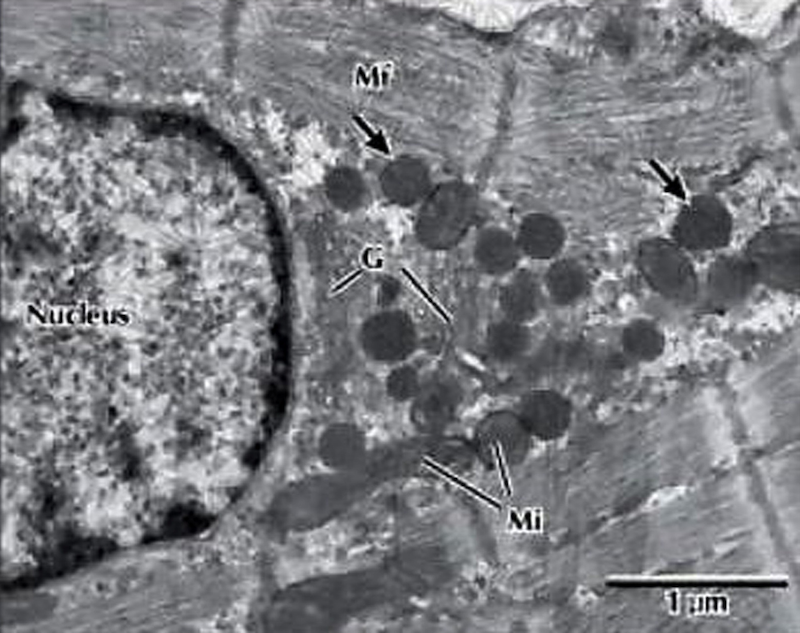
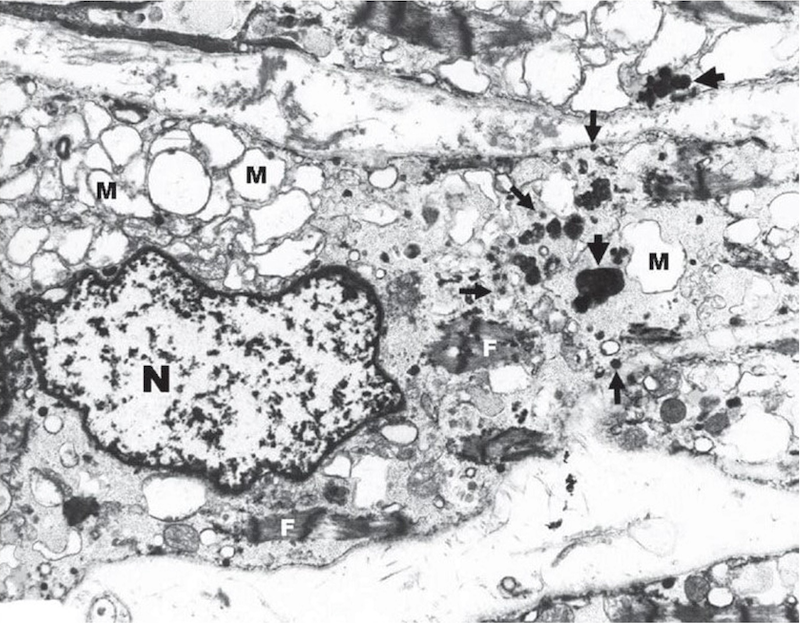
Effects of ROS in a cardiomyocyte, preclinical.
Tf-bound iron binds to TfR-1, is endocytosed and enters the cytoplasmic LIP where it is stored in ferritin or used in
cellular processes. In IOL, Tf is saturated and NTBI is detectable. NTBI enters the cell in an unregulated matter
through DMT-1, Zip 14, and LVDCC. ROS are produced, which can damage proteins, lipids and nucleic acids; proteins
damaged include myofibrils, affecting contractility, and caspases that control cell death; lipid damage can lead to cell
and organelle lysis, and alteration of membrane fluidity and channel function; mtDNA damage may decrease energy
production. The subcellular effects of ROS from excess iron on cardiomyocytes may explain the increased incidence of
clinical cardiac events in MDS1
Preclinical data demonstrate a reduction in cardiac non-heme iron levels in mice treated with L-type calcium channel
blockers2
DMT-1, divalent metal transporter 1; DNA, deoxyribonucleic acid; HSC, IOL, iron overload, LIP, labile iron pool; LVDCC,
L-type voltage dependent calcium channel; mtDNA, mitochondrial DNA; NTBI, non-transferrin bound iron; ROS, reactive
oxygen species; TfR, transferrin receptor; Zip-14, zinc transporter.
1. Kim CH & Leitch HA. Crit Rev Oncol Hematol. 2021;163, with permission; 2. Liu Q et al. American Society of Hematology
Annual Meeting 2021. Abstract 758.