Event-free survival: TELESTO
Randomized trial of deferasirox versus placebo in lower IPSS risk MDS
Event-free survival
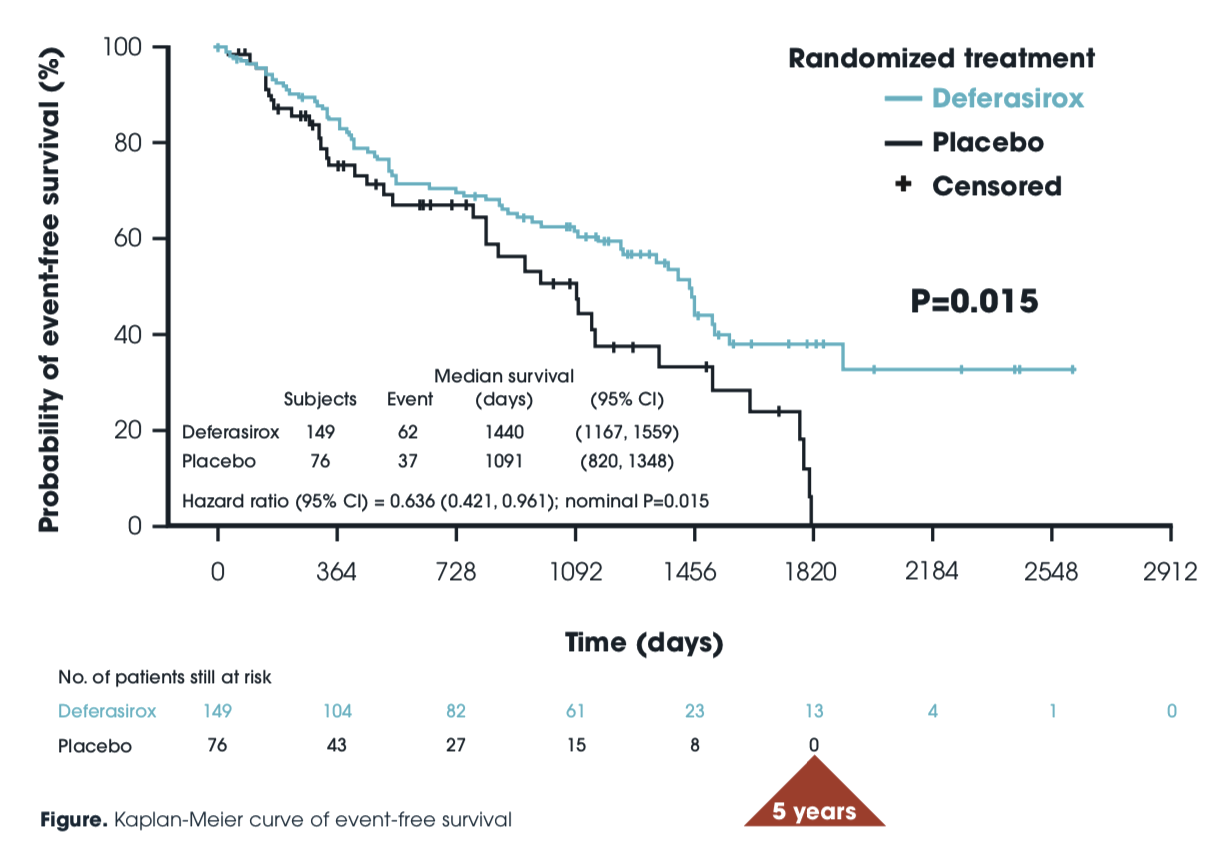
- Despite target enrollment being reduced by 2/3, making the study not powered to detect its endpoint,
- Despite half of placebo patients withdrawing from the study and subsequently receiving ICT,
- There was superior EFS in ICT patients, which became more apparent over time.
Conclusions: 1st prospective, randomized study in Low/Int-1 IPSS risk MDS with IOL showing ICT provides clinical benefit; superior EFS.
- The safety profile was consistent with previous studies of DFX in MDS.
- The results support the use of ICT in Low/Int-1-risk MDS with IOL.
Comments:
- The use of ICT after study treatment discontinuation in 1/2 of placebo patients may have diluted OS & organ event differences.
- The mean age of TELESTO patients was 61 years, younger than in other analyses2 & clinical practice (e.g. the mean ± SD age at TD in the Canadian analysis was 71.8 ± 11.3 years). This also could have diluted OS & organ event differences.
- Thus TELESTO may underestimate a clinical benefit of ICT in MDS.



