DFP
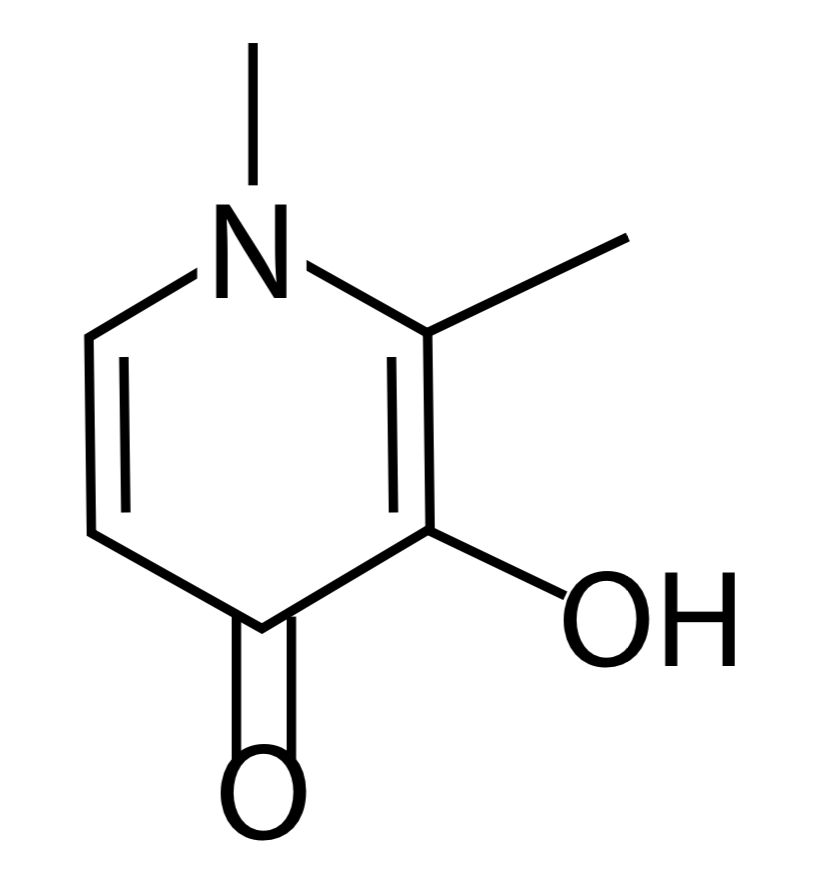
- DFP is effective at increasing iron excretion; however, its efficacy has been established chiefly in patients with hemoglobinopathies, with minimal data available in MDS.
- The safety profile of DFP in MDS is unclear, and concerns about agranulocytosis in patients with pre-existing neutropenia remain.
- In a study of 48 MDS patients receiving DFP, a 4% incidence of agranulocytosis was seen1.
- Neutrophil counts should be monitored weekly.
- The occasional MDS patient may be unable to tolerate either DFX or DFO*.
-
DFP target dose is 75mg/kg/day in 3 divided doses.
- Some patients may be able to escalate to 100mg/kg/day.
-
Common side effects of DFP are GI & arthralgias.
- Patients should be initiated on 1/5-1/4 the target dose and the dose increased gradually as tolerated.
***DFP is not Health Canada approved for use in MDS***
http://www.ferriprox.com/ca/download/ca-ferriprox-product-monograph-en.pdf
ELT
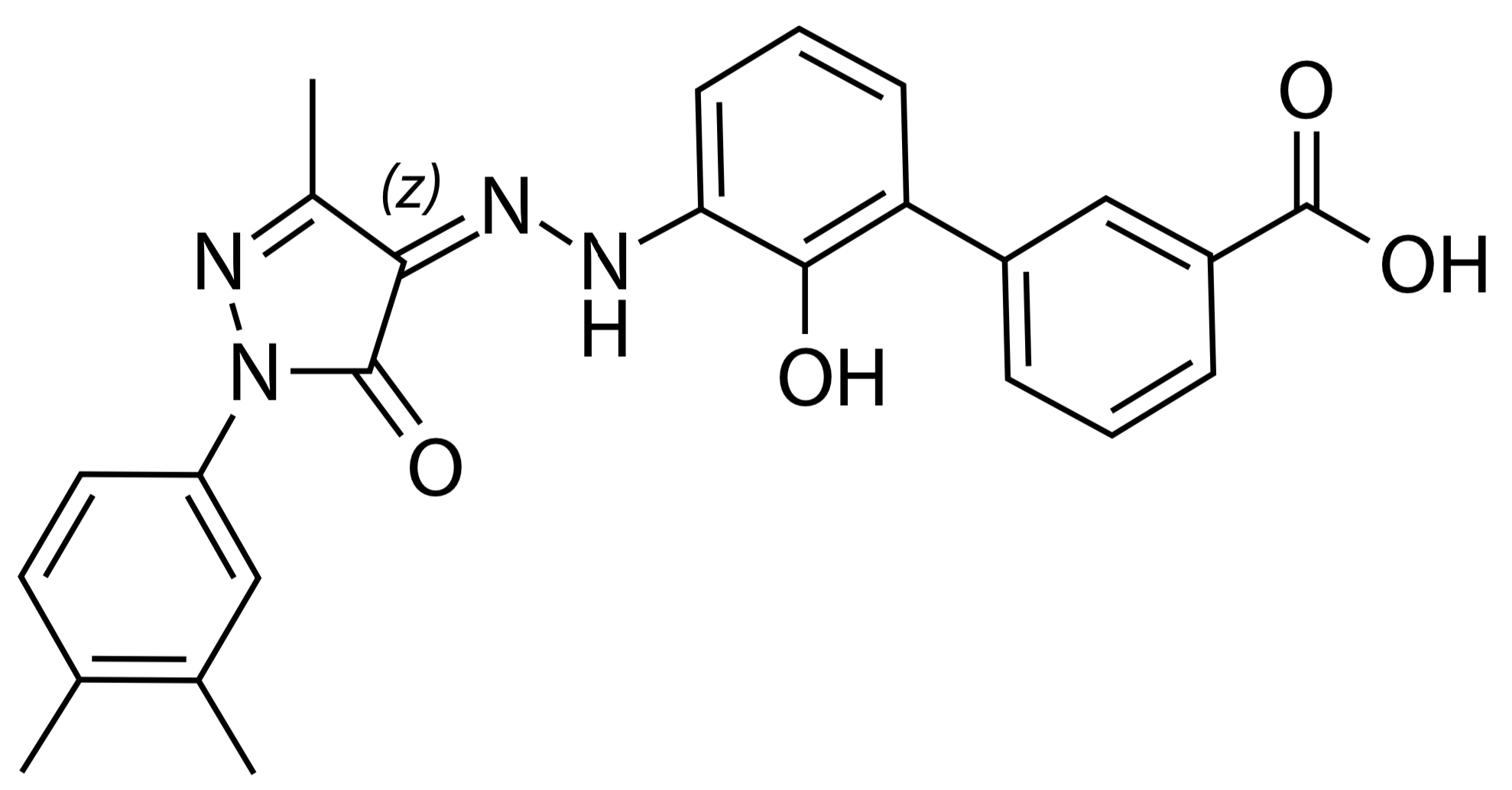
- ELT shows strong ICT activity in pre-clinical models, as a single agent or in combination with other chelators2-4.
- ***There are no clinical studies examining the ICT activity of ELT in MDS; therefore, no recommendations are made***
- A recent study suggests that ELT supports the growth and replication of hematopoietic stem cells via ICT activity5.
- A recent study indicates ELT may induce hematologic responses (all lineages) in up to 1/2 of patients with low-int-2 IPSS MDS6.
***ELT is not Health Canada approved for use in MDS***
http://www.ask.novartispharma.ca/download.htm?res=revolade_scrip_e.pdf&resTitleId=1090
Combination therapy

- Eltrombopag at low concentration in combination with other chelators offloads iron & decreases ROS production in cardiac & hepatocyte cell lines7.



